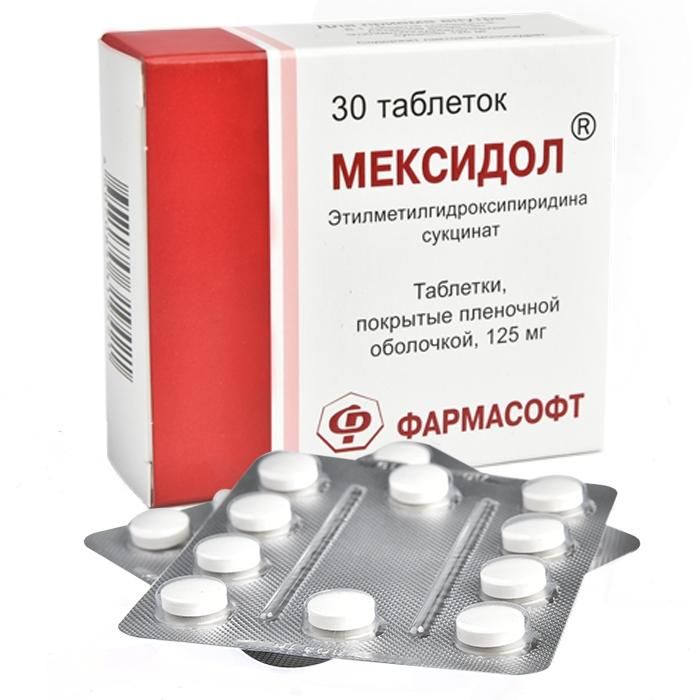
etylmetylhydroksypyrydyna | Mexidol tablets 0.125 g, 30 pcs.
Special Price
$33
Regular Price
$41
In stock
SKU
BID462422
Latin name
Mexidolum
Mexidolum
Latin name
Mexidolum
Release form
Tablets, coated with a white to white cream-colored shade, biconvex in cross section, 2 layers are visible: the inner (core) gray or creamy gray shade and the outer white or creamy white shade.
Packaging
Film-coated tablets, 125 mg. On 10 tablets in a blister strip packaging from a film of polyvinyl chloride and aluminum foil. On 1, 2, 3, 4, 5 blisters for packaging along with instructions for use in a pack of cardboard.
Pharmacological Action
Antidepressant. Selectively inhibits the reuptake of serotonin increases the concentration of the neurotransmitter in the synaptic cleft, enhances and prolongs the effect of serotonin on postsynaptic receptors. Escitalopram practically does not bind to serotonin (5-HT), dopamine (D1 and D2) receptors, β-adreno-, m-cholinergic receptors, as well as to benzodiazepine and opioid receptors.
The antidepressant effect usually develops after 2-4 weeks. after starting treatment. The maximum therapeutic effect of the treatment of panic disorders is achieved approximately 3 months after the start of treatment.
Pharmacokinetics
Absorption is independent of food intake.
Bioavailability - 80%. The time to reach Cmax in plasma is 4 hours. The kinetics of escitalopram is linear. Css is reached after 1 week. The average Css is 50 nmol / L (20 to 125 nmol / L) and is achieved at a dose of 10 mg / day. Apparent Vd - from 12 to 26 l / kg. Binding to plasma proteins - 80%.
Metabolized in the liver to active demethylated and didemethylated metabolites. After repeated use, the average concentration of demethyl and didemethyl metabolites is 28-31% and less than 5%, respectively, of the concentration of escitalopram.
The metabolism of escitalopram with the formation of demethylated metabolite occurs mainly with the participation of isoenzymes CYP2C19, CYPЗA4 and CYP2D6. In individuals with weak activity of the CYP2C19 isoenzyme, the concentration of escitalopram may be 2 times higher than in individuals with high activity of this isoenzyme.
Significant changes in the concentration of the drug with a weak activity of the CYP2D6 isoenzyme are not observed. contributes to maintaining the integrity of cardiomyocytes and maintaining their functional activity. Effectively restores myocardial contractility with reversible cardiac dysfunction.
Pharmacokinetics
Absorption
With the introduction of Mexidol in doses of 400-500 mg Cmax in plasma is 3.5-4.0 μg / ml and is achieved within 0.45-0.5 hours.
Distribution of
After i / m administration, the drug is determined in blood plasma for 4 hours The average retention time of the drug in the body is 0.7-1.3 hours.
Excretion of
It is excreted in the urine mainly in a glucuronoconjugated form and in small amounts unchanged.
Indications
consequences of acute cerebrovascular accidents, including after transient ischemic attacks, in the subcompensation phase as prophylactic courses
mild traumatic brain injury, consequences of traumatic brain injury
encephalopathy of various origins, disability mixed)
autonomic dystonia syndrome
mild cognitive impairment of atherosclerotic genesis
anxiety disorders in neurotic and neurosis-like states
states after acute intoxication with antipsychotic drugs
asthenic conditions, as well as for the prevention of stress and extreme somatic diseases) factors.
coronary heart disease as part of the complex therapy
relief of withdrawal symptoms in alcoholism with a predominance of neurosis and vegetative-vascular disorders, post-withdrawal disorders
Contraindications
hypersensitivity to the drug
acute renal failure
acute liver failure.
Due to insufficient knowledge of the effect of the drug - childhood, pregnancy, breastfeeding.
Pregnancy and lactation
There have been no strictly controlled clinical trials of the safety of MexidolВ® during pregnancy and lactation.
Composition
Active ingredient:
ethylmethylhydroxypyridine succinate (2-ethyl-6-methyl-3-hydroxypyridine succinate) - 125.0 mg
Excipients:
lactose monohydrate - 97.5 mg
povidone - 25.0 mg
magnesium stearate 2.5 mg Film coat:
opadrai II white 33G28435 - 7.5 mg (hypromellose - 3.0 mg, titanium dioxide - 1.875 mg, lactose monohydrate - 1.575 mg, polyethylene glycol (macrogol) - 0.6 mg, triacetin - 0.45 mg)
Dosage and administration
Inside, 125–250 mg 3 times a day, the maximum daily dose is 800 mg (6 tab.).
Duration of treatment - 2-6 weeks for the relief of alcohol withdrawal - 5-7 days. Treatment is stopped gradually, reducing the dose within 2-3 days.
Initial dose - 125–250 mg (1–2 tablets ) 1-2 times a day with a gradual increase until the therapeutic effect is achieved, the maximum daily dose is 800 mg (6 tablets).
Duration of therapy in patients with coronary artery disease - at least 1.5–2 months. Repeated courses (on the recommendation of a doctor), it is advisable to conduct in the spring-autumn periods.
Side effects
From the digestive system: rarely - nausea, dry mouth.
Other: rarely - allergic reactions.
The drug interaction of
Mexidol® is combined with all drugs used to treat somatic diseases.
When used together, Mexidol® enhances the effects of benzodiazepine derivatives, antidepressants, anxiolytics, antiparkinsonian and anticonvulsants.
Mexidol® reduces the toxic effects of ethanol.
Overdose
Symptoms: possible drowsiness.
Storage conditions
In a dry, dark place at a temperature of no higher than 25 РC.
Keep out of the reach of children.
Expiration
3 years.
Do not use after the expiry date stated on the package
Deystvuyuschee substances
Эtylmetylhydroksypyrydyna succinate
Pharmacy
Pharmacy terms
dosage form
Dosage form
tablets
Farmasoft, Russia
Mexidolum
Release form
Tablets, coated with a white to white cream-colored shade, biconvex in cross section, 2 layers are visible: the inner (core) gray or creamy gray shade and the outer white or creamy white shade.
Packaging
Film-coated tablets, 125 mg. On 10 tablets in a blister strip packaging from a film of polyvinyl chloride and aluminum foil. On 1, 2, 3, 4, 5 blisters for packaging along with instructions for use in a pack of cardboard.
Pharmacological Action
Antidepressant. Selectively inhibits the reuptake of serotonin increases the concentration of the neurotransmitter in the synaptic cleft, enhances and prolongs the effect of serotonin on postsynaptic receptors. Escitalopram practically does not bind to serotonin (5-HT), dopamine (D1 and D2) receptors, β-adreno-, m-cholinergic receptors, as well as to benzodiazepine and opioid receptors.
The antidepressant effect usually develops after 2-4 weeks. after starting treatment. The maximum therapeutic effect of the treatment of panic disorders is achieved approximately 3 months after the start of treatment.
Pharmacokinetics
Absorption is independent of food intake.
Bioavailability - 80%. The time to reach Cmax in plasma is 4 hours. The kinetics of escitalopram is linear. Css is reached after 1 week. The average Css is 50 nmol / L (20 to 125 nmol / L) and is achieved at a dose of 10 mg / day. Apparent Vd - from 12 to 26 l / kg. Binding to plasma proteins - 80%.
Metabolized in the liver to active demethylated and didemethylated metabolites. After repeated use, the average concentration of demethyl and didemethyl metabolites is 28-31% and less than 5%, respectively, of the concentration of escitalopram.
The metabolism of escitalopram with the formation of demethylated metabolite occurs mainly with the participation of isoenzymes CYP2C19, CYPЗA4 and CYP2D6. In individuals with weak activity of the CYP2C19 isoenzyme, the concentration of escitalopram may be 2 times higher than in individuals with high activity of this isoenzyme.
Significant changes in the concentration of the drug with a weak activity of the CYP2D6 isoenzyme are not observed. contributes to maintaining the integrity of cardiomyocytes and maintaining their functional activity. Effectively restores myocardial contractility with reversible cardiac dysfunction.
Pharmacokinetics
Absorption
With the introduction of Mexidol in doses of 400-500 mg Cmax in plasma is 3.5-4.0 μg / ml and is achieved within 0.45-0.5 hours.
Distribution of
After i / m administration, the drug is determined in blood plasma for 4 hours The average retention time of the drug in the body is 0.7-1.3 hours.
Excretion of
It is excreted in the urine mainly in a glucuronoconjugated form and in small amounts unchanged.
Indications
consequences of acute cerebrovascular accidents, including after transient ischemic attacks, in the subcompensation phase as prophylactic courses
mild traumatic brain injury, consequences of traumatic brain injury
encephalopathy of various origins, disability mixed)
autonomic dystonia syndrome
mild cognitive impairment of atherosclerotic genesis
anxiety disorders in neurotic and neurosis-like states
states after acute intoxication with antipsychotic drugs
asthenic conditions, as well as for the prevention of stress and extreme somatic diseases) factors.
coronary heart disease as part of the complex therapy
relief of withdrawal symptoms in alcoholism with a predominance of neurosis and vegetative-vascular disorders, post-withdrawal disorders
Contraindications
hypersensitivity to the drug
acute renal failure
acute liver failure.
Due to insufficient knowledge of the effect of the drug - childhood, pregnancy, breastfeeding.
Pregnancy and lactation
There have been no strictly controlled clinical trials of the safety of MexidolВ® during pregnancy and lactation.
Composition
Active ingredient:
ethylmethylhydroxypyridine succinate (2-ethyl-6-methyl-3-hydroxypyridine succinate) - 125.0 mg
Excipients:
lactose monohydrate - 97.5 mg
povidone - 25.0 mg
magnesium stearate 2.5 mg Film coat:
opadrai II white 33G28435 - 7.5 mg (hypromellose - 3.0 mg, titanium dioxide - 1.875 mg, lactose monohydrate - 1.575 mg, polyethylene glycol (macrogol) - 0.6 mg, triacetin - 0.45 mg)
Dosage and administration
Inside, 125–250 mg 3 times a day, the maximum daily dose is 800 mg (6 tab.).
Duration of treatment - 2-6 weeks for the relief of alcohol withdrawal - 5-7 days. Treatment is stopped gradually, reducing the dose within 2-3 days.
Initial dose - 125–250 mg (1–2 tablets ) 1-2 times a day with a gradual increase until the therapeutic effect is achieved, the maximum daily dose is 800 mg (6 tablets).
Duration of therapy in patients with coronary artery disease - at least 1.5–2 months. Repeated courses (on the recommendation of a doctor), it is advisable to conduct in the spring-autumn periods.
Side effects
From the digestive system: rarely - nausea, dry mouth.
Other: rarely - allergic reactions.
The drug interaction of
Mexidol® is combined with all drugs used to treat somatic diseases.
When used together, Mexidol® enhances the effects of benzodiazepine derivatives, antidepressants, anxiolytics, antiparkinsonian and anticonvulsants.
Mexidol® reduces the toxic effects of ethanol.
Overdose
Symptoms: possible drowsiness.
Storage conditions
In a dry, dark place at a temperature of no higher than 25 РC.
Keep out of the reach of children.
Expiration
3 years.
Do not use after the expiry date stated on the package
Deystvuyuschee substances
Эtylmetylhydroksypyrydyna succinate
Pharmacy
Pharmacy terms
dosage form
Dosage form
tablets
Farmasoft, Russia
Submit your review to Earn 10 Reward Points click here to login
Write Your Own Review